Covid-19 Vaccine Eligibility
Where should I sign up?
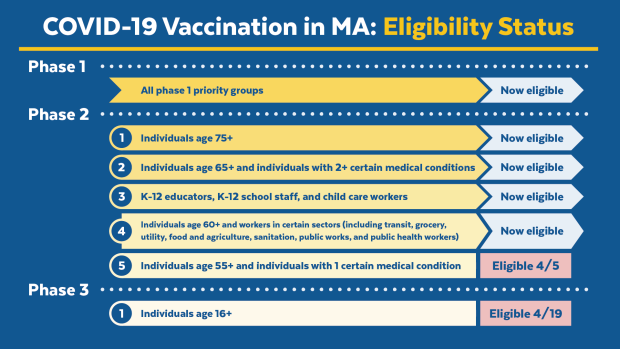
If you are eligible to get a vaccine, an appointment can be booked online. The following groups continue to be eligible for the vaccine: people age 65+, one caregiver for a person age 75+, individuals age 16+ with 2+ certain medical conditions, all groups under Phase 1.
Educators can make vaccination appointments through any of the following avenues:
- EBNHC: Prioritizing Excel staff. Call 617-568-4870 between 8AM-1PM or complete this short form so I can secure an appointment for you.
- Mass vaccination sites: March 27, April 3, April 10, and April 11 will prioritize educators. Sign up on the pre-registration system here.
- Appointments at other Massachusetts clinics (e.g. pharmacies or community centers) can continue to be made here.
Continue referring to the weekly COVID emails for updated information on where to access the vaccine.
What documentation do I need?
To get vaccinated in Phase 1 or 2, you will need to confirm you are part of the currently eligible group within those phases. To support this, all staff members received employment verification letters to use as proof of eligibility to access the vaccine. If you need a copy of your letter, please reach out to etiburcio@excelacademy.org.
If I live in another state, am I eligible for the vaccine in Massachusetts?
Individuals who live, work, or study in Massachusetts are eligible for the vaccine in Massachusetts. We will provide staff with the verification needed to access the vaccine. (Source: Mass.gov)
Which vaccine should I get? Is one better than the other?
There are now three COVID-19 vaccines authorized for emergency use: Pfizer/BioNTech, Moderna, and Johnson & Johnson. While all three have slightly different levels of efficacy (how much the vaccine lowers the risk of COVID-19) in preventing severe to moderate cases of COVID-19 (~95% for Pfizer and Moderna and 72% for Johnson & Johnson in the US, respectively), all three are close to 100% effective at preventing hospitalization and deaths. For example, no one who got the Johnson & Johnson vaccine had to go to the hospital for a COVID-19 infection after getting the vaccination. Because of this, experts recommend you get the vaccine that is available to you. Read more here to learn about efficacy and why a vaccine with higher efficacy does not necessarily mean it’s better.